I originally published this blog post on January 10th on Linkedin. Today, the XBI Biotech Index (a stock market index that tracks the performance of companies primarily involved in the biotechnology sector) crossed 100. I stand by my prediction that biotech will be one of the best performing asset classes this year. A good occasion to re-post my views on atai Life Sciences. Enjoy reading!
Almost a year ago, in March 2023, I published a blog post about why I, as atai’s founder and its largest shareholder, had increased my stake in atai.
Everything I said then IMHO still stands the test of time. I would encourage you to revisit that post if you can find the time.
2024 has started with some very exciting news, so I thought it’s time for an update.
The past year: atai was hit hard by the “biotech winter”
Since its IPO in June 2021 and through the ‘biotech winter’, atai has been in a continuous downward trend. In my opinion, the main reason is that the last 18 months have been the worst biotech has seen in more than 20 years. Rising interest rates have warranted lower biotech valuations, and with the entire sector coming from a generational high, the severe drop in valuations across biotech stocks generally created carnage and distortion. The market went from one extreme – very high valuations in early 2021 – to the other with absurdly low valuations now.
In times of uncertainty and stress like these, investors adopt a ‘risk-off’ mentality and don’t seek exposure to especially innovative companies that are pursuing novel approaches and pushing the envelope. In a risk-averse environment, investors tend to stick with traditional treatment paradigms, which is why atai (Nasdaq: ATAI), in my opinion, whose drug portfolio requires a novel view on how to treat mental health issues, has suffered one of the hardest hits.
However, I believe the biotech industry as a whole will come back with force in 2024, which should also make investors revisit atai’s groundbreaking and innovative approach to addressing the treatment of mental health conditions.
For me personally, atai is the biggest entrepreneurial opportunity I have ever encountered. I believe that this company has an incredibly bright future and is well-positioned to make a meaningful difference in addressing one of humanity’s major challenges – the escalating global mental health crisis.
Needless to say, I have never sold any stock, but have continuously (and proudly) increased my stake in atai.
Please read more about my macro view on the mental health crisis here:
· Why the total addressable market in fact is 100% of the world population
· How to stay mentally healthy in a challenging world
I am very optimistic that 2024 and the years to come will be great for atai. Since the beginning of the year, a re-rating of the stock has started, which I believe is just the beginning of a long-term change in sentiment to the better. Here is why:
1. Numbers first - atai is still trading close to cash + CMPS value
According to its most recent 10-Q filing, as of 30 September 2023, atai had approximately USD 194 million NET cash. Additionally, per its most recent 10-K , atai owns roughly 9.565m shares of Compass Pathways (Nasdaq: CMPS), which as of closing on Friday, 5 January 2024 (at closing price of USD 8.94) had a market value of roughly USD 85.5m. Hence, cash + CMPS stake alone makes USD 279.5m.
atai’s market cap as of closing on Monday, 8 January 2024, was approximately USD 335 million.
So, in other words, right now, investing in atai means investing in a company trading close to cash + CMPS and with a strong pipeline of eight (!) clinical-stage (!) mental health programs, and which (per its most recent public filings), has runway into 2026.
2. Novelty is an advantage, not a burden
The psychedelic treatment paradigm is indeed novel. It is not ‘pop a pill a day’ to put a band-aid over your struggles. It is ‘therapy combined with a psychedelic’ that aims to get to the root cause of a person’s trauma and help people truly get better.
Because of this difference in approach, I know that many investors have doubts about the rapid commercial success of psychedelic treatments once they are potentially approved. They cannot yet see the transformative potential psychedelic-assisted therapy offers for patients. I believe that if you offer outstanding value and outcomes, you will be paid accordingly. My own early discussions with insurance companies confirm my general view on the topic of reimbursement.
And then there is the ‘virality’ of psychedelic therapy: I personally know many people who have found significant healing in psychedelics – and almost all have since become advocates. Some have shared their stories only in their own private circles, while others have done so very publicly. I personally believe that, once approved, the market penetration will likely significantly outpace estimates, driven by exceptional patient outcomes and a passionate patient advocacy movement.
3. For patient access: the shorter, the better
When it comes to mental health, there is no ‘one size fits all’ solution. A suite of psychedelic-based treatment options will be needed to address the varieties of patient populations even within indications, such as depression. atai wants to provide that toolbox to therapists, so that they can decide which psychedelics might fit best the needs of the respective patient.
I believe that short-duration psychedelics - those that elicit psychedelic experiences of around or under two hours – will be especially important in driving access and scale. This is why I am excited by atai’s decision to expand its focus early-on. atai started with DMT already in 2019 and has now enlarged its portfolio to other shorter-duration psychedelics like 5-MeO-DMT and psilocin (more below).
Data to date suggests that short-duration psychedelics could offer clinical benefits comparable to longer-duration psychedelic compounds, but with shorter treatment times and reduced medical resource requirements. Additionally, I support atai’s belief that the two-hour interventional treatment window that has already been established by J&J’s SPRAVATO (esketamine) could potentially be leveraged for the commercial roll-out of short-duration psychedelics - if approved - in the future.
4. Strategic investment into Beckley Psytech
In this context, I am very excited to tell you more about atai’s recently announced strategic investment in Beckley Psytech, a private biopharmaceutical company focused on transforming short-duration psychedelics into clinical treatments.
atai acquired 35.5% of Beckley Psytech through a total investment of USD 50 million. In addition, atai received a 1:1 warrant coverage with a 30% premium on the primary issuances and has the right to appoint and hold three of nine seats on Beckley Psytech’s Board of Directors. If atai were to exercise its warrants, it would come close to owning 50% of Beckley Psytech.
Importantly, atai will hold a time-limited right of first refusal on any future sale of the company, asset sales, or other transfer of commercial rights, as well as an indefinite right of first negotiation for BPL-003 and ELE-101, highlighting the potential for an even closer collaboration of these two firms in the future.
(Closing celebration dinner with the legendary Amanda Feilding and her son Cosmo, CEO of Beckley Psytech.)
Since its founding in 2018, atai has always had the ambition to be ‘THE’ psychedelics company. The psychedelic mothership, so to speak.
With the acquisition of the stake in Beckley, atai continues to cement and strengthen its position as the leading company focused on the renaissance of psychedelics globally, with a portfolio that I believe encompasses all major psychedelics with therapeutic use cases – what I call the Big Five, so to say:
· Psilocybin (& psilocin)
· DMT
· 5-MeO-DMT
· MDMA
· Ibogaine
While I take great pride in atai being one of the first companies to bring psychedelics back to the medical world in this century, we are indeed standing on the shoulders of giants who have been advocating for their therapeutic use since the 1960s.
Beckley’s founder, Lady Amanda Feilding, is one of these giants. Joining forces with Amanda, her family and her team makes me especially proud for atai.
5. Beckley adds two best-in-class drug candidates
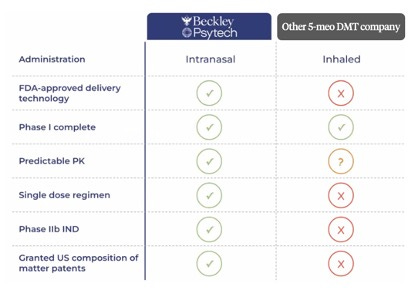
The stake in Beckley brings BPL-003 (intranasal 5-MeO-DMT) and ELE-101 (intravenous psilocin) onto atai’s mental health innovation platform.
BPL-003 and ELE-101 are on-track for multiple anticipated clinical readouts within the next 12 months, including a Phase 2b readout of BPL-003 in treatment resistant depression (TRD), anticipated in the second half of 2024.
BPL-003 is a patent-protected, intranasal, dry powder formulation of 5-MeO-DMT. Three trials are currently ongoing with BPL-003, with a Phase 2b trial actively recruiting in the US under an IND that was accepted by the FDA in February of last year. Compared to other ways of administering 5-MeO-DMT, we believe the Beckley version is far superior.
ELE-101 is a patent-protected, intravenous formulation of psilocin, which is the active moiety in the body when psilocybin is administered orally. As such, we view ELE-101 as a relatively de-risked asset for its stage of development, as it leverages the robust proof-of-concept data that has been established with psilocybin across multiple trials.
It is currently being developed for the treatment of Major Depressive Disorder (MDD) and has the potential to offer the therapeutic benefits of psilocybin in a more consistent, controllable, and shorter (!) treatment paradigm of less than 2 hours. Initial results from the current ELE-01 Phase 1/2a study are anticipated in the first half of 2024.
6. Strong news flow pipeline
As I said earlier, I am very excited for 2024. Firstly, because I firmly believe the biotech market will come back with force and gusto. But more importantly, I’m excited because atai anticipates plenty of inflection points.
7. R-MDMA is the new rising star in atai’s portfolio
In addition to the acquisition of the stake in Beckley, atai reported very good news for R-MDMA last week.
Fully in line with one of my life’s missions to ‘spread the love’, atai has been working on a new, improved version of MDMA over the past few years. As you might know, MDMA has shown strong therapeutic potential, especially in PTSD, and approval by the FDA of MAPS’ MDMA product is expected in 2024.
Last week, atai reported positive results from its Phase 1 study evaluating orally administered EMP-01, the R-enantiomer of MDMA. As stated in its most recent 10-K, atai has a range of patents pending on this moiety, as well as on derivatives and prodrugs of (R-)MDMA.
The goals of this Phase 1 study were to evaluate the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of EMP-01. EMP-01 was well-tolerated, and treatment-related adverse events (AEs) were all expected and generally dose dependent. There were no study discontinuations, and no serious or severe adverse effects were observed in the study.
Even better, EMP-01 administration resulted in a differentiated subjective experience compared to racemic MDMA on standard psychedelic experience questionnaires. Furthermore, dose dependent changes on measures of emotional breakthrough, a phenomenon thought to be a key mediator of the long-term psychological changes associated with psychedelics, were noted in this healthy volunteer population.
Building upon the decades of research into MDMA as a potential treatment for mental health disorders, including two positive Phase 3 studies in PTSD, I believe the unique characteristics of EMP-01 are very encouraging and look forward to exploring the implications for further clinical development.
8. A rich pipeline of novel psychedelics
I see the world of clinical psychedelics in three stages:
· First movers: These are Psilocybin (Compass) and MDMA (MAPS). atai is delighted to support their trailblazing efforts by being the largest single shareholder of Compass and as a donor to MAPS. They are doing the hard work, truly paving the way.
· Short-duration: The second generation will be imo shorter-duration psychedelic products that combine known short-acting psychedelic moieties - DMT, 5-MEO-DMT, and Psilocin—with proprietary formulation technologies that result in psychedelic experiences with rapid onset, a duration of 30-45 minutes, and resolution within 90 minutes.
· Third-generation: And then you have the third generation, which are completely novel compounds, optimizing to reduce side effects, treatment effects and practical matters like duration. atai’s R-MDMA program is the perfect example, and atai has a very rich pipeline of more of these third generation psychedelics coming up via its discovery engines.
Ibogaine stands out and is separate, being a very long duration psychedelic of more than 24 hours, requiring a clinical stay of 2-3 days. But in return, Ibogaine has the potential to be ‘disease modifying’ in substance-use-disorders, even in the most severe forms of this condition like opioid addiction for which existing therapies leave much to be desired.
9. And there’s more…
While I personally regard psychedelics as the most important and promising tool in fighting the mental health crisis, there are also very interesting non-psychedelic compounds that can play a role in supporting mental health. In its most recent public flings, atai has two such non-psychedelic drug development programs:
RL-007, a Pro-Cognitive Neuromodulator for Cognitive Impairment Associated with Schizophrenia (in phase 2b) and GRX-917, Deuterated Etifoxine for Anxiety Disorders (ready for phase 2).
You can find more info about those 2 programs in my 2023 blog post and on the atai website.
I personally believe these two non-psychedelic drugs are ‘gems within a goldmine’.
10. Being an atai shareholder makes the world a better and happier place
Over the years, many philanthropists have decided to support scientific research via non-profits. While I am a huge believer in the immense value of scientific research, when it comes to psychedelics, I believe thanks to decades of this scientific work there is now a robust evidence base to show that they work in treating mental health disorders.
Now it is all about bringing those treatments to the patients!
For this, we need clinical trials such as those that atai, Beckley, and Compass Pathways are running. The funding needed to bring psychedelics through the full FDA approval process is too big for non-profit work. A clear testament of this is the transformation of MAPS, the mother of all psychedelic non-profits, to a for-profit model under its new name, Lykos Therapeutics. This is the model needed to bring potentially transformative new treatments to patients successfully, safely, and quickly.
I am an investor in atai not just because of my conviction that it will pay off financially in a massive way, but because I believe in making the world a happier and better place.
The future is bright
I hope you can feel my excitement about the potential of atai. And I am not alone. atai is covered by 9 research analysts. The price targets of those analysts’ range between USD 6 and USD 21 per share.
I want to thank all fellow shareholders who have gone through the ups and downs of the atai share price together with me. As an investor, I am very confident in the potential of atai and am convinced that me and my fellow investors will be rewarded – with performance, and with knowing that we will have made a transformational difference for people suffering from mental health issues.






But 9.565m shares of Compass Pathways (Nasdaq: CMPS), which as of closing on Friday, 21 February 2024 (at closing price of USD 4.21) had a market value of just USD 40.3m.